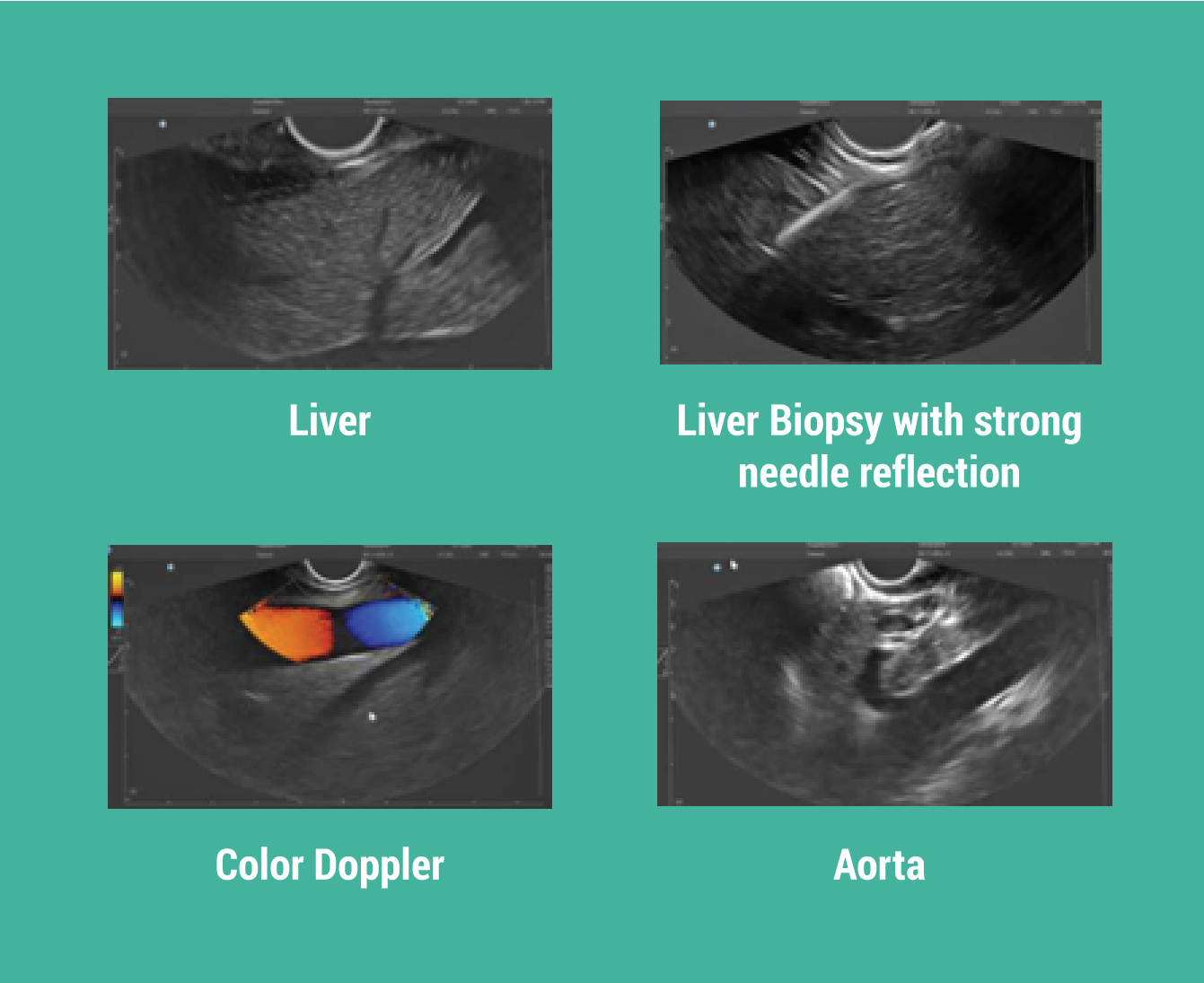
PORTLAND—July 20, 2021—

Oregon-based medical device innovator, EndoSound®, Inc., announced today the addition of Josh Cohn to its executive leadership team as the company’s first Chief Commercial Officer. Additionally, the company announced it successfully raised Initial Seed Round funding as it oversubscribed its intended goal. The new funding will be used to commercialize the EndoSound Vision SystemTM.
In this role, Josh Cohn reports to Stephen Steinberg, Founder and President, and is responsible for the pre- and post-commercialization Sales and Marketing strategies. Based in Charlotte, North Carolina, Josh will collaborate with world-renowned endoscopists and prioritize bringing the novel EndoSound endoscopic ultrasound platform to market.
Josh comes with extensive experience in the gastrointestinal endoscopy device market, most recently acting as a Market Development Manager at Ambu A/S. Previously, he spent 13 years at PENTAX Medical, where he held several roles including National Sales Director and Regional Sales Director. While at Ambu A/S and PENTAX Medical, Josh was responsible for the creation of new sales and marketing programs, including customer retention and conversion initiatives.
“I’m ecstatic to join EndoSound as it approaches a critical milestone in its growth. Expanding healthcare access and diminishing infection risk while reducing costs for patients fills me with pride,” stated Josh. “The EndoSound Vision System is truly revolutionary and presents a safer, more affordable alternative to conventional EUS platforms.”