Portland, OR, (June 18th, 2024) — EndoSound®, Inc., is thrilled to announce that the Centers for Medicare & Medicaid Services (CMS) has granted a Transitional Pass-Through (TPT) code, effective July 1, 2024, for the companies EndoSound Vision System™, or EVS™, a revolutionary advancement in endoscopic ultrasound (EUS) technology.
The CMS TPT status is monumental as it provides outpatient facilities with an incremental Medicare payment for procedures in which the EVS is utilized. This status is granted to new and innovative medical devices that meet eligibility criteria. Pass-through payments are intended to help facilitate patient access to new technologies. Details on the TPT code, C1606, can be found in the July 2024 update of the hospital outpatient prospective payment system, which is accessible on the CMS website.
“We are incredibly proud to receive the TPT status from CMS for the EVS,” said Scott Aldrich, CEO of EndoSound. “This approval underscores the clinical value and innovative nature of our technology. It enables broader access to the benefits of EUS, which is critical for early detection and treatment of gastrointestinal diseases. Our goal is to improve patient outcomes while reducing healthcare costs, and this milestone brings us a big step closer to achieving that.”
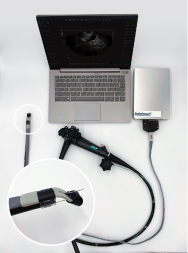
The EVS is an innovative, cost-effective solution designed to enhance the capabilities of existing endoscopic equipment, providing high-resolution ultrasound imaging without the need for expensive and complex new scopes. This breakthrough technology promises to improve the accessibility and quality of care for patients undergoing EUS procedures.
EndoSound remains committed to advancing medical technology and improving patient access to care.
About EndoSound
EndoSound, Inc., is a privately held company based in Portland, Oregon. EndoSound is dedicated to expanding access to endoscopic ultrasound technology around the world. With its patented design, EndoSound transforms any flexible upper endoscope into a fully functional EUS scope. EndoSound is comprised of an experienced team of scientists and engineers, clinicians, and seasoned business professionals with a track record of bringing new medical devices to market.
To learn more about the EVS, please visit endosound.com/endosound-vision-system
For media inquiries, please contact:
Patrick Hurley
VP of Marketing
pr@endosound.com










 PORTLAND—August 31, 2021—Endosound, Inc., an Oregon-based medical device innovator developing technologies that enhance access, reduce cost, and increase the safety of endoscopic ultrasound procedures, announced today that its EndoSound Vision System received a Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA).
PORTLAND—August 31, 2021—Endosound, Inc., an Oregon-based medical device innovator developing technologies that enhance access, reduce cost, and increase the safety of endoscopic ultrasound procedures, announced today that its EndoSound Vision System received a Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA).