Case Study: Windsock diverticulum
Introduction:
Echo-EGD, which I define as diagnostic EGD enhanced with ultrasound, is perhaps ideal to maximize the find rate. Today, it is more accessible than ever and with it comes the promise to improve patient care and reduce healthcare spending.
We believe that Echo-EGD is more possible and necessary than ever because:
- There are 8 million EGDs performed annually in the U.S.
- A significant portion could benefit from a more comprehensive exam compared to standard EGD.
- It can often reduce the need for additional cross-sectional studies such as CT or MR.
- Echo-EGD can be performed in the ASC.
High equipment costs have confined EUS mainly to hospital settings, limiting access. The EndoSound Vision System™ (EVS™) technology transforms any endoscope into a fully functioning EUS system. EVS is cost-effective and opens new possibilities for Echo-EGD across care settings.
Below I describe a case where Echo-EGD with EVS helped achieve a definitive diagnosis.
Patient History
A 50-year-old male with reflux and dysphagia was found to have a subepithelial lesion in the second part of the duodenum during EGD. The patient was referred for EUS for further investigation.
The Procedure
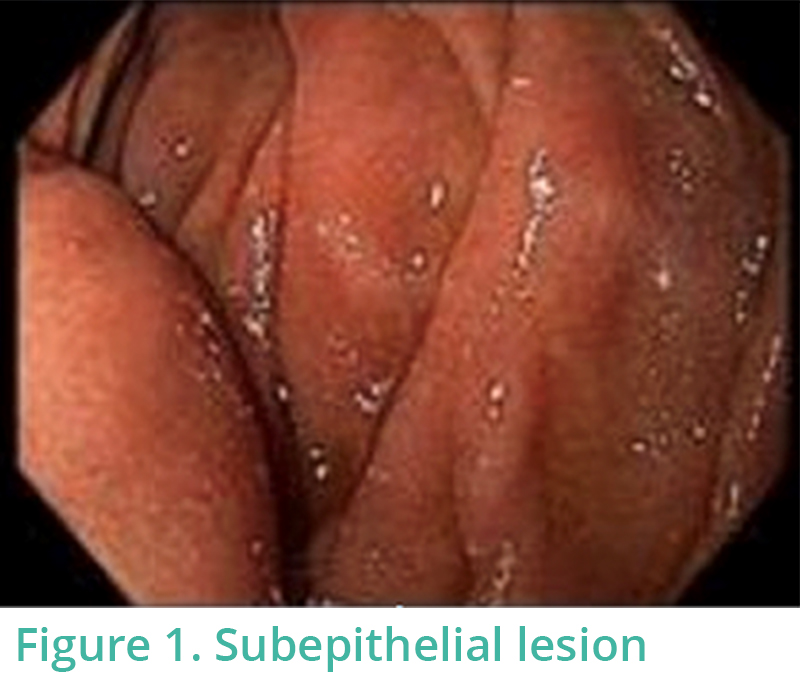
A subepithelial lesion was identified in the second portion of duodenum (Figure 1). Proximal to this lesion, there was a diverticulum with a large opening (Figure 2). To have an optimal angle for endosonography based on the endoscopic location of the lesion, the ultrasound probe was placed at 7 o’clock position (Figure 3). EUS confirmed that the “subepithelial lesion” seen on EGD was the outer portion of a windsock-like diverticulum. Water infusion in duodenal lumen resulted in water flowing into the inner part of diverticulum on EUS (Figures 4, 5). Figure 5 shows the sonographic image of a windsock diverticulum as seen on EUS with the picture of an actual windsock inserted for comparison.




Conclusion
Confirmation of a windsock diverticulum masquerading as a subepithelial lesion was achieved by performing EUS with EVS. Even though this case was a referral from another gastroenterologist, it could have easily been an Echo-EGD case should the lesion have been found by an endosonographer with access to EVS at the time of initial EGD. This, in turn, would have enabled the delivery of efficient care cost-effectively. Another advantage of EVS compared to traditional EUS scopes is that the probe placement can be tailored to the location of the lesion viewed endoscopically. In our case, the lesion was in 7 o’clock position and thus the probe was placed at 7 o’clock position accordingly. Lastly, the forward viewing scope orientation reduced the risk of accidentally entering the diverticulum and causing perforation – a complication occasionally seen with side-viewing endoscopes.
References:
Sahai et al., Gastrointest Endosc 2000;52:153-9. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia.
Chang et al., Gastrointest Endosc 2010; 72: 967-74. EUS compared with endoscopy plus transabdominal US in the initial diagnostic evaluation of patients with upper abdominal pain.

